|
Chemistry Class - XII 1999
(CBSE)
You are on questions 21 to 34 of Set I
Q.21 Account for the following : (2
marks)
(i) Alkylamines are stronger bases than arylamines.
(ii)
Toluene is more easily nitrated than benzene.
Q.22 (i) Why
do the transitional metals slow variability in their oxidation
state?
(ii) Write formula of a compound where the transitional metal is
in +7 oxidation state. (2 marks)
Q.23 Draw the structure and write the hybridisation
state of the central atom of each of the following species :
(i)
Fe(CO)5
(ii)
trans-[CO(NH3)4Cl2]+ (Atomic
Number : Fe = 26, CO = 27) (2 marks)
Q.24 What happens during glycolysis and what are the
products of glycolysis ? (2 marks)
Q.25 What are antibiotics? Name any two antibiotics
which are specific for certain diseases. (2 marks)
Q.26 How are Buna-S and Terylene synthesised? Give
chemical equations.
(2 marks)
Q.27 When 0.532 g of benzene (C6H6), boiling point
of 353 K, is burnt with excess of oxygen in a constant volume system, 22.3
kJ of heat is given out. Calculate H for the combustion process. (R=8.31 J
K-1 mol-1)
(3 marks)
Q.28 Account for the following :
(i) Of the
lanthanide's, cerium (atomic number 58) forms tetrapositive ion, Ce4+ in
aqueous solutions.
(ii) Transition metals have high enthalpy of
atomisation.
(iii) Scandium forms no coloured ions and yet it is
regarded as a transition element. (3 marks)
Q.29 Explain giving reason for each of the following
:
(i) Chloroaceticacid has higher pKa value than acetic acid.
(ii)
Carboxylic acid have higher boiling points than the alcohol's of
comparable molecular masses.
(iii) Electrophilic substitution in
benzoic acid takes places at meta position.
(3 marks)
Q.30 (i) Explain the meaning of the statement,
"Absorption is a surface phenomenon."
(ii) State two features of
chemical adsorption which are not found with physical adsorption. (3
marks)
Q.31 (i) What is photosynthesis? Where does it occur
in plants?
(ii) Name two products of photosynthesis which are required
for the survival of most of the chemotrophs. (3 marks)
Q.32 Complete the following nuclear reactions
:
(i)
9642Mo(.....,n)9743Tc
(ii)
..........(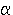 ,
2n)21185At ,
2n)21185At
(iii) 24696 +
126 --> ...... + 4(10n) (3
marks)
Q.33 (a) Account for the following :
(i)
Haloalkanes are more reactive than haloarenes.
(ii) Phenols are acidic
in nature.
(iii) Unlike phenols, alcohol's are easily
protonated.
(b) Write the reactions involved in the preparation of
:
(i) 1, 2-ethanediol from ethene, and
(ii) iodoform from
2-propanol (5 marks)
Q.34
Present a comparative account of the following :
(i) Physical states of
nitrogen and phosphorus.
(ii) Maximum covalency numbers of oxygen and
sulphur.
(iii) Bond energies of F2 and Cl2.
(iv) Structures of
chlorides of boron and aluminium.
(v) Proton affinities of NH3 and PH3
(5 marks)
|
|
